Menene titanium dioxide?
Babban bangaren na titanium dioxide shine TiO2, wanda shine muhimmin pigment na markarwa a cikin nau'i mai tsananin ƙarfi ko foda. Ba mai guba bane, yana da babban farin farin ciki da haske, kuma ana ɗaukar mafi kyawun farin launi don inganta fararen fata. Ana amfani dashi sosai a masana'antu kamar coatings, roba, roba, tawada, tawada, birerciyoyi, gilashi, da sauransu.

Ⅰ.Titanium dioxide masana'antu masana'antu:
(1) Upstream na masana'antar masana'antu dioxide ya ƙunshi kayan masarufi, ciki har da Ilmenite, titanium mai daure, da sauransu;
(2) Midstream yana nufin samfuran dianium dioxide.
(3) Downstream filin aikace-aikacen Titanium Dioxide.Titanium dioxide ana amfani dashi sosai a fannoni daban daban kamar coatings, faruruwa, takarda kai, tawada, roba, da sauransu.

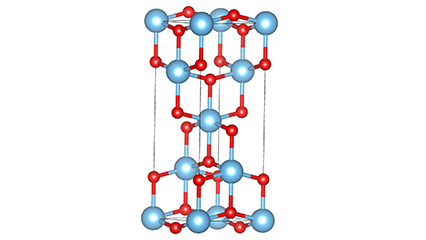
Ⅱ.The Tsarin Crystal na Titanium Dioxide:
Titanium dioxide wani nau'in fili ne na polymorphous, wanda ke da cikakken bayani guda uku cikin yanayi, wanda aka shine Anatase, Rutile da Brooke.
Dukkanin rutile da anatase suna cikin tsarin Tetragonal, wanda ke tsaye a ƙarƙashin zazzabi na yau da kullun; Brookite na tsarin orthorhombic, tare da tsarin kristal din ba tare da ingantaccen darajar ba, saboda haka ba shi da ƙima mai mahimmanci a masana'antar a yanzu.

Daga cikin tsarin ukun, lokaci mai wahala shine mafi barga guda. Lokaci na anatase zai canza canzawa cikin lokaci mai wahala sama da 900 ° C, yayin da tsararren lokaci ba zai canza ba a cikin lokaci mai gudana sama da 650 ° C.
(1) m aiki titanium dioxide
A cikin rutile m m dioxide, ti atoms suna a tsakiyar lattice na Cirten, da oxygen zarra suna located at sasanninta na titanium-oxygen Ocaledron na titanium-oxygen Ocaledron. Kowane octahedron an haɗa shi da 10 kewaye octahedrons 10 (ciki har da keɓaɓɓen ra'ayoyi biyu na gefuna biyu), da kwayoyin biyu TOO2 biyu suna samar da kwayoyin halitta guda biyu.
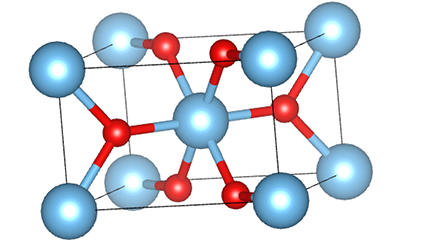
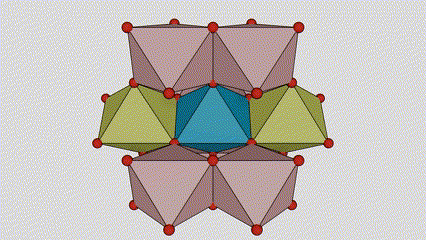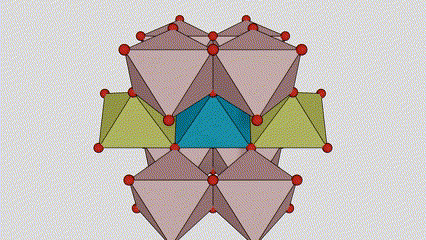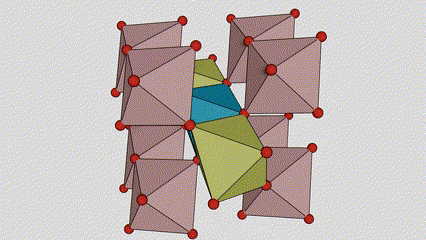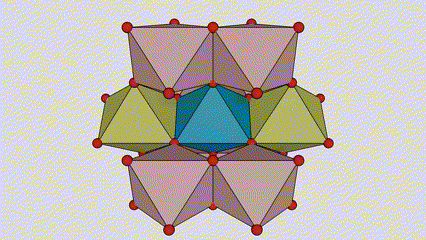
Tsarin zane na Crystal Crystal Titanium Dioxide (hagu)
Hanyar haɗin Titanium Optaledron (dama)
(2) Atime Atze Titanium Dioxide
A cikin Aatase Azimeum Dioxide, kowane titanium-oxygen Octaideron an haɗa da 8 kewaye Ocehedrons 4 Raba kwayoyin cuta (4 raba kwayoyin cuta 4), da kwayoyin halitta 4 na TiO2), suna samar da kwayoyin halitta 4.
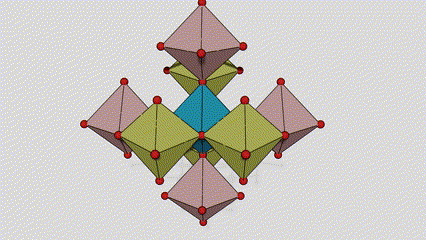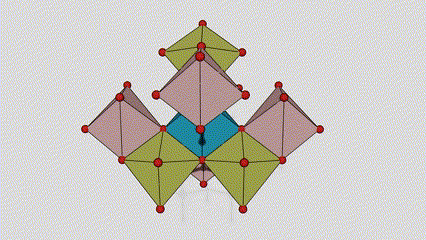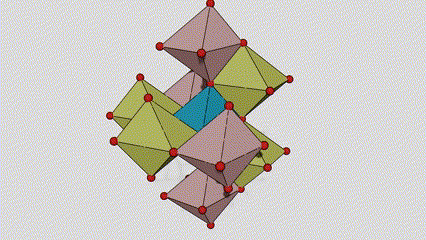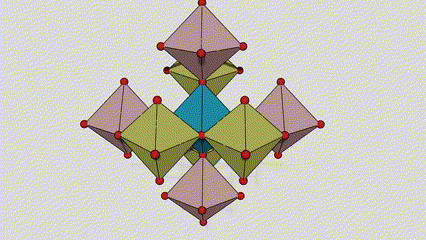
Tsarin zane na Crystal Crystal Titanium Dioxide (hagu)
Hanyar haɗin Titanium Optaledron (dama)
A Hanyar Titanium Dioxide:
Tsarin samarwa na titanium dioxide yafi haɗa da tsarin acid da aiwatar da aikin yi.

(1) tsari na acid
A acid tsarin samar da titanium dioxide ya ƙunshi acidlysis na titanium baƙin ƙarfe tare da mai da hankali sulfurical don samar da titanium sultanis. Bayan lissafawa da murkushe, titanium dioxide samfuran aka samo. Wannan hanyar na iya samar da anatase da kayan marmari dioxide.
(2) aiwatarwa
Tsarin cinikin titanium dioxide ya shafi hadawa da ruhun ko cokali sannan kuma aiwatar da choke chaces don samar da titanium tetrachloride. Bayan isasshen iskar-shaye-zafin-kai, ana samun samfurin iskar titanium dioxide ta hanyar filltration, Wanke ruwa, bushewa, da murƙushewa. Babban cinikin titanium dioxide na iya samar da samfuran ruhun.
Yadda za a bambance amincin Titanium Dioxide?
I. Hanyoyi na zahiri:
(1)Hanya mafi sauki ita ce kwatanta irin zane ta taɓa. Karya titanium dioxide yana jin smoother, yayin da gaske na gaske dioxide ji rougher.

(2)Ta hanyar rinsing da ruwa, idan ka sanya wasu titanium dioxide a hannunka, karya daya yana da sauƙin wanka, yayin da gaske mutum bashi da sauƙin wanka.

(3)Aauki kopin ruwa mai tsabta da sauke titanium dioxide a ciki. Wanda ke iyo zuwa ga farfajiya shine na gaske, yayin da wanda ya daidaita zuwa kasan karya ne (wannan hanyar bazai aiki ba don samfuran da aka kunna ko gyara abubuwa).
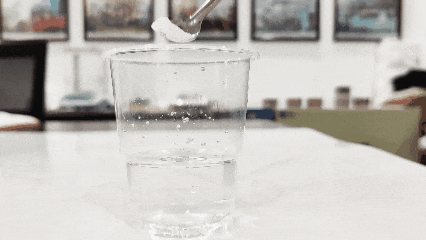

(4)Duba kararwarsa cikin ruwa. Gabaɗaya, titanium dioxide yana narkewa a cikin ruwa (ban da titanium dioxide musamman faranti, tawada, wanda ke da ban mamaki a cikin ruwa).

II. Hanyoyin sunadarai:
(1) Idan an kara alli foda acid: ƙara hydrochloric acid zai haifar da haɗuwa mai ƙarfi tare da sauti mai yawa (saboda calbonate carbonate ya amsa da acidi dioxide).

(2) Idan an ƙara Lithpone: ƙara girki sulfuric acid ko hydrochloric acid zai samar da wani ɓataccen kwai wari.

(3) Idan samfurin hydrophawic, ƙara hydrochloric acid ba zai haifar da amsa ba. Koyaya, bayan shafa shi da ethanol sannan a ƙara hydrochloric acid, idan an samar da kumfa ta ƙunshi samfurin carbonated carbonate foda.

III. Haka kuma akwai wasu hanyoyi guda biyu:
(1) ta amfani da dabara PP + 30% gf + 5% pp-g-mah + 0.5% titanium dioxide, da mafi ingantaccen titanium dioxide.
(2) Zabi resin mai haske, kamar yadda m Abs da 0.5% titanium dioxide kara da aka kara. Auna hasken sa watsawa. Thearamar hasken wutar lantarki shine, mafi ingantaccen gidan titanium dioxide shine.
Lokaci: Mayu-31-2024





